Eur. J. Inorg. Chem – Isabelle Gerz, Dr. Sergio Augusto Venturelli Jannuzzi, Dr. Knut T. Hylland, Dr. Chiara Negri, Dr. David S. Wragg, Dr. Sigurd Øien-Ødegaard, Prof. Mats Tilset, Prof. Unni Olsbye, Prof. Serena DeBeer, Prof. Mohamed Amedjkouh.
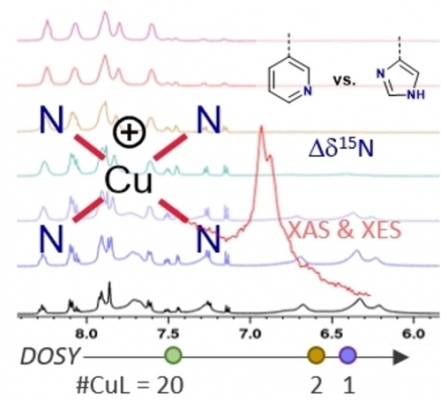
A series of Cu(I) complexes of bidentate or tetradentate Schiff base ligands bearing either 1-H-imidazole or pyridine moieties were synthesized. The complexes were studied by a combination of NMR and X-ray spectroscopic techniques. The differences between the imidazole- and pyridine-based ligands were examined by 1H, 13C and 15N NMR spectroscopy. The magnitude of the 15Nimine coordination shifts was found to be strongly affected by the nature of the heterocycle in the complexes. These trends showed good correlation with the obtained Cu-Nimine bond lengths from single-crystal X-ray diffraction measurements. Variable-temperature NMR experiments, in combination with diffusion ordered spectroscopy (DOSY) revealed that one of the complexes underwent a temperature-dependent interconversion between a monomer, a dimer and a higher aggregate. The complexes bearing tetradentate imidazole ligands were further studied using Cu K-edge XAS and VtC XES, where DFT-assisted assignment of spectral features suggested that these complexes may form polynuclear oligomers in solid state. Additionally, the Cu(II) analogue of one of the complexes was incorporated into a metal-organic framework (MOF) as a way to obtain discrete, mononuclear complexes in the solid state.
Click here for the complete article!