J. Am. Chem. Soc. – Blaise L. Geoghegan, Yang Liu, Sergey Peredkov, Sebastian Dechert, Franc Meyer, Serena DeBeer, and George E. Cutsail III
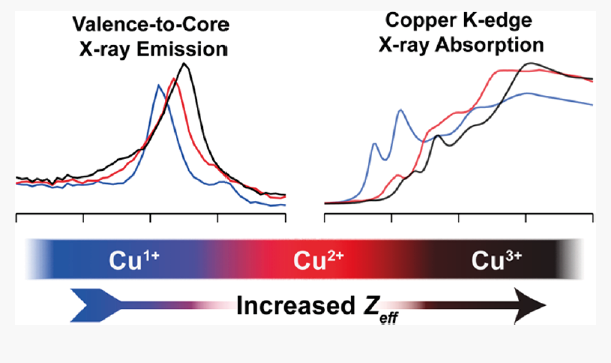
A series of organometallic copper complexes in formal oxidation states ranging from +1 to +3 have been characterized by a combination of Cu K-edge X-ray absorption (XAS) and Cu Kβ valence-to-core X-ray emission spectroscopies (VtC XES). Each formal oxidation state exhibits distinctly different XAS and VtC XES transition energies due to the differences in the Cu Zeff, concomitant with changes in physical oxidation state from +1 to +2 to +3. Herein, we demonstrate the sensitivity of XAS and VtC XES to the physical oxidation states of a series of N-heterocyclic carbene (NHC) ligated organocopper complexes. We then extend these methods to the study of the [Cu(CF3)4]− ion. Complemented by computational methods, the observed spectral transitions are correlated with the electronic structure of the complexes and the Cu Zeff. These calculations demonstrate that a contraction of the Cu 1s orbitals to deeper binding energy upon oxidation of the Cu center manifests spectroscopically as a stepped increase in the energy of both XAS and Kβ2,5 emission features with increasing formal oxidation state within the [Cun+(NHC2)]n+ series. The newly synthesized Cu(III) cation [CuIII(NHC4)]3+ exhibits spectroscopic features and an electronic structure remarkably similar to [Cu(CF3)4]−, supporting a physical oxidation state assignment of low-spin d8 Cu(III) for [Cu(CF3)4]−. Combining XAS and VtC XES further demonstrates the necessity of combining multiple spectroscopies when investigating the electronic structures of highly covalent copper complexes, providing a template for future investigations into both synthetic and biological metal centers.
Click here for the complete article!