Journal of Catalysis – Kelsi R. Hall, Carlotta Pontremoli, Tom Z. Emrich Mills, Fabrizio Careddu, Morten Sørlie, Matteo Bonomo, Claudia Barolo, Vincent G. H. Eijsink, Silvia Bordiga
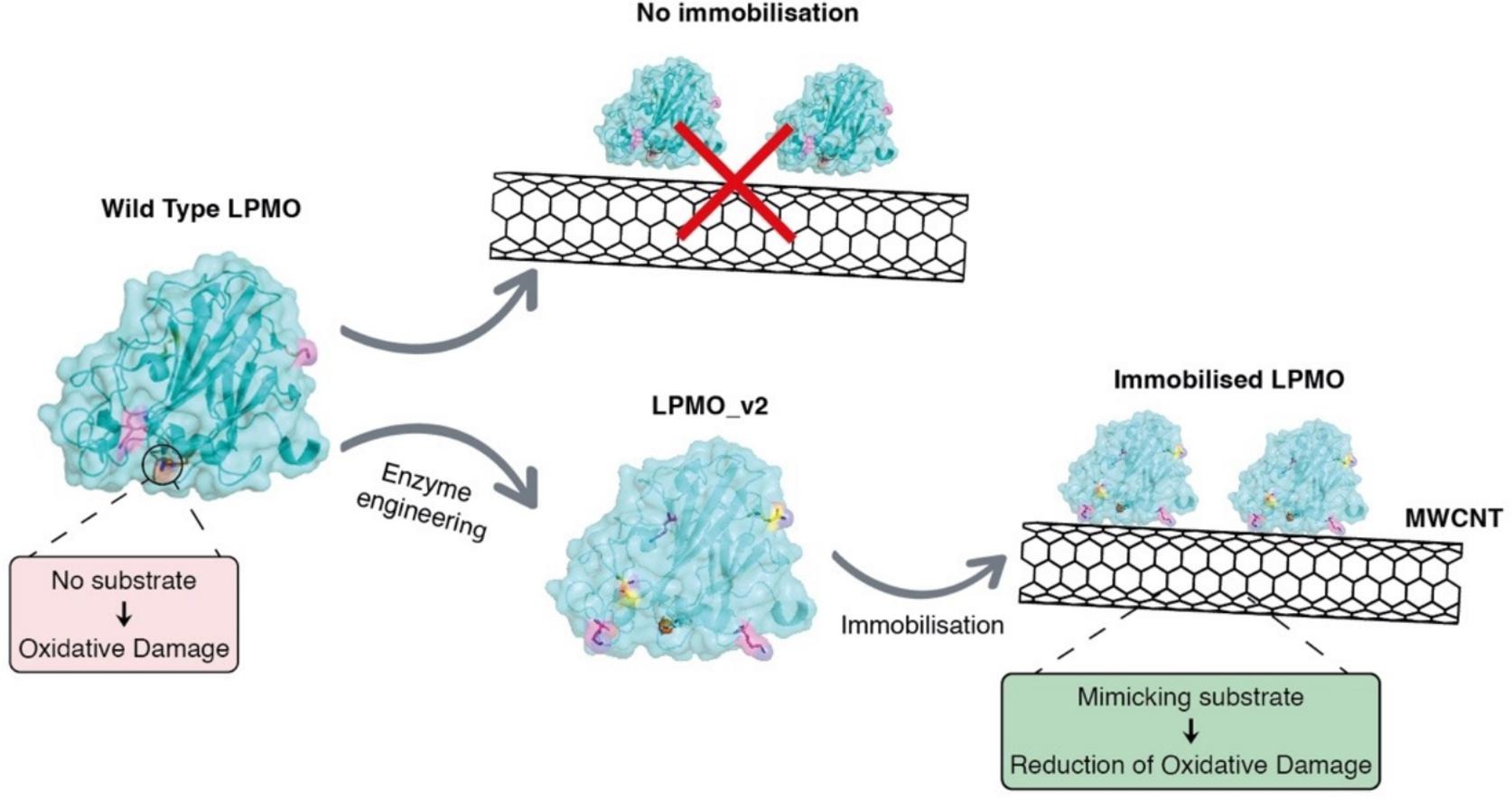
Lytic polysaccharide monooxygenases (LPMOs) are mononuclear copper-containing enzymes that are able to oxidise C–H bonds in the glycoside linkages of polysaccharides. However, LPMOs are prone to oxidative damage, particularly in the absence of an adequate substrate. In this work, we investigated whether we could immobilise LPMOs and whether such immobilisation could enhance the stability of LPMOs while preserving the essential catalytic properties of the copper active site. Two LPMOs from different families, LsAA9A and ScAA10C, were selected and immobilised on carboxylic acid functionalised multiwalled-CNTs, using a two-step carbodiimide activation reaction. To improve the frequency of enzyme immobilisation and guide site-specific orientation, the enzymes were engineered, introducing two lysine residues on two different loops on the LPMO surface. Assessment of the oxidase and peroxidase activities of the LPMO-MWCNT bioconjugates showed that immobilisation of the engineered LPMO was much more efficient compared to the wild-type enzymes. The immobilised enzymes still showed activity on several substrates, confirming retained catalytic competence following immobilisation. Incubation of the free and immobilised LPMOs under damaging conditions indicated a protective effect of immobilisation for LsAA9A-MWCNT, indicating that, for some LPMOs, immobilisation on MWCNTs may protect against oxidative damage.
Click here for the complete article!