Dalton Transactions – Barbara Centrella, Gabriele Deplano, Alessandro Damin, Matteo Signorile, Mariagrazia Tortora, Claudia Barolo, Matteo Bonomo and Silvia Bordiga
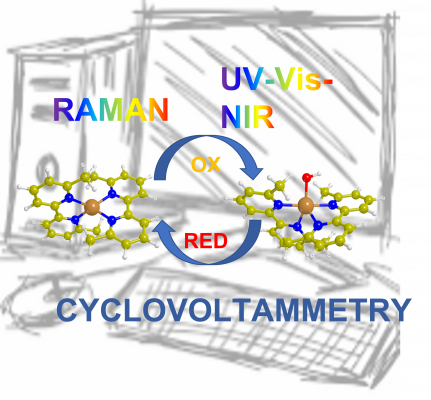
The effect of differently substituted 2,2’-bipyridine ligands (i.e. 6,6’-dimethyl-2,2’-bipyridine, 5,5’-dimethyl-2,2’-bipyridine, 6,6’-dimethoxy-2,2’-bipyridine and 2,2’-bipyridine) on the reversible oxidation of the resulting CuI homoleptic complexes is investigated by means of a multi-technique approach (electronic and vibrational spectroscopies, DFT, electrochemistry). Among the four tested complexes, CuI (6,6’-dimethyl-2,2’-bipyridine)2 shows a peculiar behavior when oxidized with an organic peroxide (i.e. tert-butyl hydroperoxide, tBuOOH). The simultaneous use of UV-Vis-NIR and Raman spectroscopy methods and cyclovoltammetry, supported by DFT based calculations, allowed identifying (i) the change in the oxidation state of the copper ion and (ii) some peculiar modification in the local structure of the metal leading to the formation of a [CuIIOH]+ species. The latter, being able to oxidize a model molecule (i.e. cyclohexene) and showing the restoration of the original CuI complex and the formation of cyclohexanone, confirms the potential of these simple homoleptic CuI complexes as model catalysts for partial oxygenation reactions.
Click here for the complete article!